In drug development, pharmacokinetic (PK) assays give us information on the fate of the drug (e.g., distribution, absorption and/or secretion) following its administration to an animal or human. This analysis is often carried out alongside pharmacodynamic (PD) assays that provide information about a drug’s pharmacological effect on the body.
As such, PK assays are a key component of the drug development process with the global PK assay service market currently valued at US $652.8 million (link is external). PK assay results are critical in all phases of drug development, starting from target/molecule selection during the discovery stage, through PK projections from animal to human to inform doses for early clinical studies, to safety and efficacy evaluation during proof-of-concept and confirmatory clinical trials (Phase II/III). Numerous decision-making milestones in drug development programs rely, at least in part, on PK assay results and the exposure-response relationships based on PD endpoints or clinical endpoints. Increasingly companies (link is external) are using data from drug monitoring assays at all stages of the development pipeline to enhance the productivity and efficiency of new drug development processes.
Both PK and PD assays require critical reagents (e.g. specific anti-drug antibodies) that can specifically and accurately measure the drug in plasma and tissues. Sensitive and reproducible PK assays are required as part of the complex and lengthy development process for new therapeutic molecules. These drug monitoring assays must be well characterized, fully validated, and documented in order to yield reliable results compliant with the necessary regulatory standards.
Antibody PK assays
Particular challenges arise in developing drug monitoring assays for therapeutics when the molecule itself is a human or humanized monoclonal antibody. This is due to the excess of human antibodies in patient serum. In such cases, the antibody PK assay reagents must be highly specific to the antibody therapeutic to prevent cross-reactivity with the innate antibodies in human samples. As antibody therapeutics are now the fastest growing class of pharmaceuticals identifying the required critical PK assay reagents to allow accurate and efficient development of these biologics is vital.
Which form of antibody to measure?
Due to the bivalent nature of antibodies, in the presence of their target they can exist in different forms including fully bound, partially bound or unbound forms. To properly interpret the biotherapeutic concentration and characterise PK in these drug monitoring assays it is important to know which form of the drug antibody is being measured.
At low circulating target concentrations or if the antibody therapeutic is maintained at higher concentrations than the target the total and free drug levels can be considered equivalent. However, with high circulating target, both total and free versions of the antibody therapeutic can coexist. The development of anti-drug antibodies by the immune system in response to treatment can further complicate antibody PK assay results by binding the drug and impacting PK measurements.
Typically, PK scientists are interested in free antibody as the pharmacologically active form of the drug and thought to give a better correlation with efficiency. Yet, toxicologists may be more interested in determining total drug levels in samples, to interpret drug exposure and the potential safety implications. Consequently, the specific reagents used in the PK assay to the various forms of antibody found in clinical samples should be determined by the desired end point of the assay.
The most commonly used platform for antibody PK assays are ligand binding assays. Recent guidance from the ligand-binding assay (LBA) bioanalytical focus group (LBABFG) from the American Association of Pharmaceutical Scientists (AAPS) (link is external)suggests the form of antibody measured in the drug monitoring assay should depend upon the disease biology and the specific drug’s mode of action.
Improving antibody PK assay development
Quantification of PK for antibody therapeutics is an essential part of drug development. Poor PK results of therapeutics is one of the key reasons for drug attrition from development pipelines. In order to reduce concerns about high drug attrition, researchers are embracing the trend of investigating antibody PK assay results of novel biologics at each development stage. PK readouts are largely determined by ligand binding assay quality and format. Assay quality can be impacted by many factors, such as availability and quality of ligand binding assay reagents, as well as limited assay development timelines. Often, the best assay strategy is assumed to be selection of the best performing assay using the available reagents within the required timeframe to prevent expensive and unnecessary delays to the drug development process.
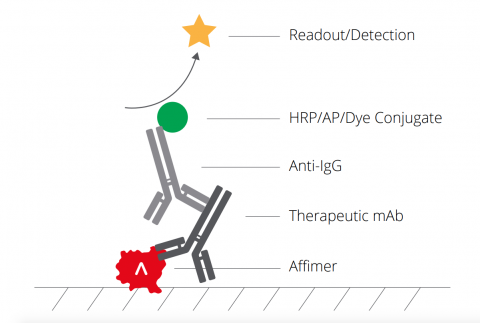
In this respect, the development of ligand binding assay reagents, such as Affimer binders, offers an ideal solution to antibody PK assays. Affimer binders to antibody therapeutics are highly target specific and sensitive, have a proven 13 week development period to prevent delays in project timelines, and can be easily produced without batch-to-batch variation. Validation of these reagents by the leading CRO, Covance, shows that they meet regulatory requirements for critical reagents in PK assays.
Taking a broader view, bioanalytical validation via antibody PK assays is a cornerstone of not only PK and clinical pharmacology, but also in clinically-based decision making. If you don’t have confidence in the data, and its collection, then how can you make rational dosing decisions based on the collected data? If you’re not able to make rational dosing decisions, then how can you determine with any degree of certainty the safety and efficacy of a drug product? In this light, opting for high quality critical reagents for antibody PK assays is an essential first step to accelerate the effectiveness and efficiency of antibody drug development.