From antibodies and antibody fragments to nanobodies and synthetic antibodies – affinity reagents, it seems, are shrinking.
Monoclonal antibodies were viewed as the gold standard capture reagent utilised by researchers for decades. Though they have been used for the purposes of protein detection, quantification and inhibition across a range of different assays, their widespread use within the field of biological research is not necessarily testament to their functionality.
Numerous reports now highlight both cross-reactivity and reproducibility issues with these molecules. In addition, the development of monoclonal antibodies is both expensive and time-consuming, often stalling projects before they’ve started. Furthermore, at 150kDa the large size of these molecules limits their use in certain applications, particularly where affinity reagents may compete for space in the recognition of closely juxtaposed epitopes. These limitations of monoclonal antibodies have proven insurmountable for many researchers.
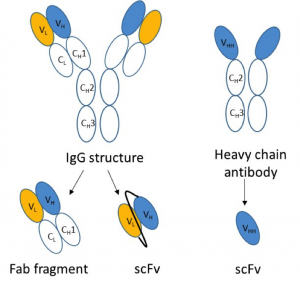
Antibodies and antibody derived fragments have all been used as protein capture reagents.
To overcome some of these issues, particularly that of size, antibody fragments have been utilised as antibody alternatives. Fab fragments and short chain variable fragments (scFv) are both affinity reagents based on the antibody structure. Fab fragments, with an approximate molecular weight of 50kDa, are formed from the Fab arms of an antibody, containing one constant and one variable region of both the light and heavy chains. A second wave of antibody fragment technology produced the smaller 27kDa scFv, which consists of the antibody heavy and light chain variable regions connected via a short linker.
As both Fab fragments and scFv retain the antigen-binding site of the variable regions, they inherit the specificity and affinity of the intact antibody. While the reduction in size of the scFv over the Fab fragment offers increased penetration of tissue and access to hidden epitopes, they are less stable in storage than the larger Fab fragments. For both antibody fragment types the removal of the stabilising Fc receptor portion of the antibody means that the circulating half-life of these molecules in vivo is very short and as custom antibody fragment services are not as readily available they have not driven the required change in research reagents in the field of protein science.
The discovery of camelid antibodies offered even smaller capture reagents to protein studies. Camelid antibodies are naturally occurring heavy chain only antibodies, the N-terminal fragment of which is termed a VHH domain or nanobody and can act as a small affinity reagent just half the size of a scFv. From the available data these VHH domains appear to be superior in binding to both antibody fragments and traditional IgG antibodies. Though they exhibit good binding characteristics and the required small size, unfortunately, much of the technology is covered by patents, preventing widespread uptake of this technology, particularly within the research field.
More recently synthetic antibodies have been developed that offer researchers protein capture reagents with the required high target affinity, rapid development times and small molecular weight of a similar size to that of camelid antibodies. With molecular weights in the region of 8-25kDa this class of affinity reagent follows the trend for smaller affinity reagents and offers rapid production times of up to 12 weeks. Both nucleic acid and protein molecules have been developed as synthetic antibodies with varying levels of success, though the major focus of manufacturers of these molecules has been on the therapeutic market. Within the synthetic antibody market custom antibodies seem to be the norm, with people turning to these molecules when antibodies have already failed them.